Introduction
Open recirculating cooling systems are widely used by companies in the Netherlands to dissipate released process heat. Via heat exchangers the heat released during process flow is transferred to the cooling water. The heated cooling water is then cooled and reused. Fresh water is continuously added to the system. In the larger installations, the necessary drainage flow is in most cases discharged untreated.
Due to the higher temperatures, cooling water provides ideal conditions for the growth of micro-organisms. In addition, particles and lime can adhere to the installation, and corrosion can occur. These processes can have a negative impact on the efficiency of the cooling water system.
To prevent these unwanted processes, additives are added to the cooling water system. Examples are biocides to combat the growth of (micro) organisms, corrosion inhibitors to prevent rust, and anti-scaling products to prevent scale deposits. In addition to these products, other products can also be added. Think, for example, of
buffers for regulating the pH values.
The variety of products, their use, and the water hazard of these products can vary greatly. In most cases, cooling water is discharged into the surface water untreated. It is unclear whether and, if so, to what extent these discharges have consequences for the quality of surface water. The results of the study “Acute Toxicity of Cooling Water Discharges from Recirculation Systems” (Baltus, 1999) show that in addition to biocides, other additives to the cooling water system may also influence the toxicity of the cooling water. One of the recommendations from the study is to investigate the toxicity of the other additives used in open recirculating cooling systems. To gain more clarity on this, a study was carried out on behalf of Rijkswaterstaat (RWS) into the use of all additives in open recirculating cooling systems at a number of companies in the Netherlands. The results of this are described in this report.
The aim of this research is to provide insight into the total use of additives in open
recirculating cooling systems in the Netherlands. The sub questions are:
- Which additives are used?
- What is the function of these additives?
- What is the scope of the use of the different types of additives?
- What amounts of additives are discharged into surface water?
- What is the (possible) impact on the quality of the surface water?
The use of additives from twelve companies was investigated for this study. The
recirculating cooling systems of these companies combined are representative of
the average large recirculating cooling systems in the Netherlands in terms of size,
additives used, and continuity of the systems.
- Background Information and Research Design
1.1 Open Recirculating Cooling Systems
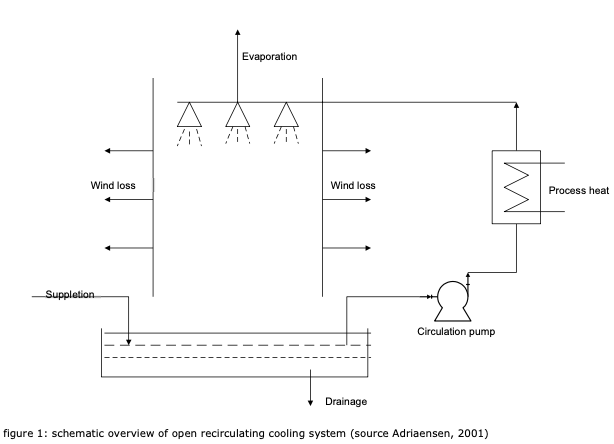
There are hundreds of companies in the Netherlands that discharge cooling water from an open recirculating cooling system into the surface water. In an open recirculating cooling system, heat from the processes is transferred to the cooling water via heat exchangers. The cooling water releases the absorbed heat via evaporation in a cooling tower or condenser (Adriaensen et al. 2001). The salt concentration increases due to the evaporation of the water. In addition, contact with the air also increases the concentration of floating parts. To reduce thickening of the water, the water is replaced by suppletion water, and the water is drained from the system.
1.2 Company Selection
In consultation with water quality managers, RWS WVL (Water Traffic and Living Environment) approached companies with the request to cooperate in this research. In addition, the advisory files of two companies were requested. In order to determine whether the cooling water system of a company is sufficiently representative for this research, the following requirements were set in advance:
- is it an open recirculating cooling system?
- is the size of the cooling water system of sufficient size to form a representative image?
- has the cooling water system been in operation for some time and continually?
- does the company keep track of the discharge flow, or can it be determined which maximum flow has been licensed?
- does the company keep track of which additives are used and in what quantities?
To ensure the confidentiality of the companies that participated in this investigation, the company names are not mentioned, instead the companies have been given a code (1 to 12). Company 1 has two open recirculating cooling systems that discharge directly into the surface water. These have been referred to in this study
as 1a and 1b.
The total flow rate of all industrial open recirculating cooling systems in the Netherlands is not accurately known. Roughly speaking, the selected twelve companies are jointly responsible for approximately 30% of the total flow.
1.3 Use of Additives
The data regarding the use of the additives by the companies in this study has not been noted and recorded for later use. However, data is available on the quantities of products purchased per year.
For this study the purchase quantities were added up for the period 2014–2017 and divided by the number of years, since purchase and consumption can differ. The average is considered representative for the consumption of the specific product per year in this study. The usage data for 2018 has not been included in this study. The reason for this is that at some companies at the time of requesting, the data was
not yet available.
Data on additives used by companies (product name and quantity) has been collected. In most cases this consists of a safety data sheet (MSDS) provided by the companies or suppliers, or easily available through the supplier’s website. In some cases, the suppliers of the products have submitted the safety data sheets to us.
In addition, recent discharge flows of the cooling water were collected from a number of companies. If the flow rate was not known or could not be traced, the maximum permitted discharge flow rate, as is stated in the water permit, is assumed.
1.4 Considerations and Uncertainties
The maximum discharge flow is likely to be an overestimate of the actual flow. Statements by the water quality managers show that the maximum annual flow rate was not exceeded by the companies in the period 2014 to 2017. This means that the concentration of the ingredients in the cooling water is probably higher in reality.
The concentration of an ingredient in an additive is in most cases indicated in a certain range (for example 10-20%). This study is based on the highest concentration mentioned. This slightly corrects the possible overestimation of the flow rate. It is expected that, as a result of this consideration, the calculated concentrations of substances in the cooling water will reasonably correspond to the actual concentrations.
In some cases not all ingredients are listed on a safety data sheet of a product. This is because ingredients that are less than 1% present (0.1% in the case of substances of very high concern) do not have to be included in the ingredient list. Obtaining, analyzing and processing ingredient lists of all additives from suppliers would in all likelihood greatly slow down the progress of this research, and was not done for this reason.
This study assumes that all additives are dosed continuously. In reality, biocides in particular will have been administered intermittently. This causes the actual concentration of these substances in the cooling water to fluctuate.
2. Details of Additives, Function, Scope of Use, and Water Hazard
A total of 66 different cooling water additives were used in open recirculating cooling systems by the companies. With the exception of one product, the safety data sheets and/or substance data of these products has been collected. This concerns BT3801 (pH buffer). This product was not included in this study. Many additives contain multiple ingredients. These are categorized into the following groups.
- phosphorus compounds
- polymers
- oxidative biocides
- non-oxidative biocides
- acids and bases
- corrosion inhibitors
- descaling agents
- de-chlorinating agents
- antiscalants (soaps)
- anti-foaming agents
- unknown/other resources
This chapter describes the data regarding substances, toxicity, and the scope of use. An overview of all additives is included as appendix 2.
2.1 Phosphorus Compounds
Phosphates and phosphonates are highly soluble, non-bioaccumulative, poorly fully degradable substances. In open recirculating cooling systems, these substances are mainly used as corrosion inhibitors. Phosphorus compounds bind magnesium and calcium ions from the water and partly deposit on a metal surface. This creates a protective layer against corrosion.
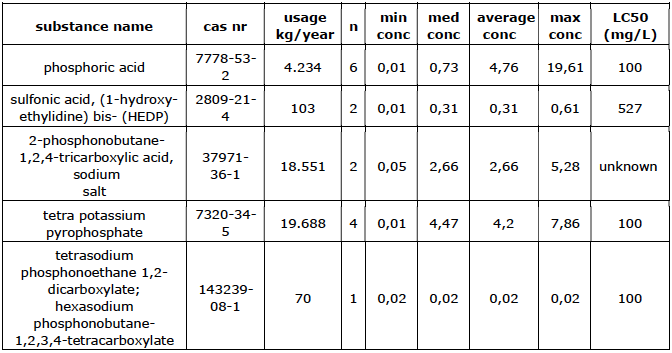

of times detected, med = median, conc = concentration in mg/L.
The total usage of phosphorus compounds is 49,260 kg/year (approximately 8.5% of the total use of additives). The estimated median concentrations in the cooling water range from 0.001 mg/L to 7.5 mg/L per company. One of the possible causes of the large spread is that the effectiveness can vary per substance.
The phosphorus compounds listed in Table 1 are generally of little harm to aquatic organisms, but the toxicity is not always known. It is also unclear whether these substances can break down in the surface water into substances with a higher water hazard. For example, some phosphonates can be converted to the poorly degradable substance aminomethylene phosphonic acid (AMPA) (Kalf D.F. et. Al, 2002).
2.2 Polymers
Polymers are mainly used in cooling water systems as a dispersant and/or hardness stabilizer. The molecular weight of a polymer ranges from 500 to 70,000 grams/mol.
Usage overview

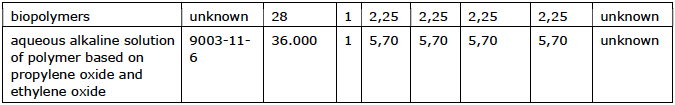
Polymers are poorly biodegradable; the longer polymer chains in particular break down very poorly (Ulrich et al. 2001). For example, the degradation of the copolymer of acrylic acid and maleic acid with a molecular weight of 70,000 grams/mol is 20% after 30 to 90 days. At a molecular weight of 1,000 grams/mol, 45% is degraded in the same period. The aquatic ecotoxicity of polymers is generally low. The EC50 of the acrylic and maleic copolymer for crustaceans and fish exceeds 200 mg/L.
Polycarboxylates and anionic acrylamide are not absorbed by aquatic organisms. The reasons for this are the size of the molecules and the hydrophilic properties of the substance.
The use of polymers compared to other additives in cooling water systems is large. The results of this study show that the 12 companies jointly use 261 tons of polymers per year, which is approximately 45% of the total of additives used by the companies. The majority of the polymers will eventually end up in surface water via the effluent. It is striking that the estimated concentration of polymers in the effluent differs considerably per company (range 0.22 mg/L to 197 mg/L).
2.3 Oxidative biocides
To control biological growth in open recirculating cooling systems, oxidative biocides are applied both intermittently and continuously (R.P.M. Berbee, 1997). Different types of oxidative biocides for cooling water systems are available.
Oxidative biocides convert various compounds through oxidation. During the dosing of hypochlorite (and other hypochlorite based oxidative biocides), a large part is broken down by decomposition processes of organic matter in water (R.P.M. Berbee, 1997). The concentration of free chlorine in the cooling water will only increase once the easily oxidized substances have been oxidized, and halogenated nitrogen compounds have been formed and subsequently broken down again. The effluent concentration calculated here is only based on the amount of oxidative biocides purchased. Therefore, the calculated concentrations of sodium hypochlorite are higher than the concentrations of free chlorine normally measured in the cooling water.
Six companies use two different types of oxidative biocides, active chlorine and BCDMH. One company also uses sodium bromide in combination with active chlorine. This enhances the effect of sodium hypochlorite. The use of oxidative biocides amounts to a total of 83,277 kg/year (approximately 14.6% of the total use of additives).
Usage overview of oxidative biocides

Sodium hypochlorite
This substance has an optimal activity at a pH between 6.0 and 7.5 and is effective up to a pH of 9.0. Use at a lower pH is not recommended due to corrosion formation (Baltus, 1996). This biocide is the most commonly used oxidative biocide in the 12 selected companies (over 99%). The calculated dosed concentrations of sodium hypochlorite in the cooling water vary widely per company (0.04 mg/L to 16.80 mg/L).
Sodium hypochlorite is quickly converted in cooling water. It can thus be seen to a certain extent as “readily biodegradable”. About 1% of the dosed sodium hypochlorite is converted into various haloforms (chloroform, bromoform and mixtures thereof) as a by-product. Some of these substances will evaporate in the
cooling towers. In that case the use of sodium hypochlorite will have less adverse effects on the quality of surface water (Berbee R.P.M., 1997). In addition to haloforms, bromamines and the harmful bromate can also form in cooling water. Bromate is a substance of very high concern. It should be noted that hypochlorite
can contain 34 to 37 mg of bromate per kg.
The estimated median concentration of sodium hypochlorite in the companies is 2.40 mg/L. Presumably, in most cases sodium hypochlorite is applied intermittently, so the actual amounts used will be higher. The ratio of sodium hypochlorite to free chlorine that is used depends on several factors. This study assumes that the average use of sodium hypochlorite by the companies corresponds to the concentrations of free chlorine found in the study of Acute Toxicity of Cooling Water Discharges (Baltus, 1999) (2.0 mg/L) and the recommended values from the report of Aqua Nederland (0.5 mg/L to 5.0 mg/L) free chlorine (Adriaensen, 2001).
In one company, the average concentration of sodium hypochlorite is 16.80 mg / L. It is suspected that sodium hypochlorite has been dosed continuously at this company. Depending on breakpoint chlorination conditions, the use of sodium hypochlorite at these high concentrations may result in undesirably high
concentrations of haloforms, bromates and other harmful substances in surface water.
When using BCDMH, the product will be broken down into HOCl and HOBr. This substance has almost the same effect as sodium hypochlorite. In high concentrations, this substance can have adverse effects on the quality of surface water. The data from Aqua Nederland (Adriaensen, R. 2001) shows that the optimal
maintenance dose of free chlorine varies between 3 mg/L and 10 mg/L. Company 6 uses this substance. However, the calculated average concentration is low (0.24 mg/L). The product is probably used incidentally in higher concentrations than calculated here.
Water hazard
Advantages of using this substance over sodium hypochlorite is that the HOBr present is a stronger oxidant than sodium hypochlorite. Bromamines are also effective as an oxidant. As a result, less product is required for optimal operation.
Sodium bromide/HOCl
This substance is effective only in combination with sodium hypochlorite (Adriaensen, R. 2001). The oxidative effect is comparable to that of BCDMH.
Sodium bromide is used by company 2. The average concentration of sodium hypochlorite used (0.4 mg / L) in the cooling water at this company is lower than the average use of the companies where sodium hypochlorite is used without sodium bromide.
2.4 Non-Oxidative Biocides
These biocides have a more selective effect than oxidative biocides. Microorganisms are killed by damaging the cell membrane, disrupting the transport of substances in the cell, leaking cell components, or hindering energy production (Anonymous, 1991). Non-oxidative biocides often require a longer activity and reaction time than oxidative biocides.
Only one of the researched companies used a non-oxidative biocide (see Table 4).
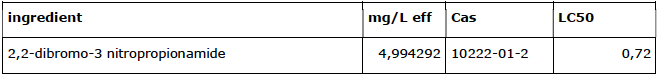
Usage overview of non-oxidative biocides2
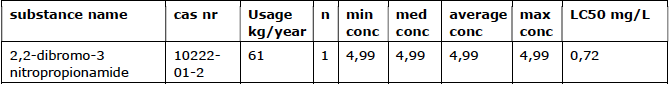
med = median, conc = conc. in mg/L. Product is approved by Ctgb as Novocide 30
The peak concentration is probably higher in practice because DBNPA has to be administered intermittently (conditions of use Ctgb).
2,2-dibromo-3 nitropropionamide (DBNPA) is converted in the cooling system into two substances: DBNPA-1 and DBNPA-2 (Baltus C.A.M., 1999). The degradation rate depends on temperature and pH. Chapter 3 examines the behavior of DBNPA
and other non-oxidative biocides in cooling systems in more detail. DBNPA is a very strong, acutely toxic substance (LC50 <1 mg/L).
The total use of non-oxidative biocides in this one company is 61 kg/year. This is approximately 0.01% of the total.
2.5 Acids and Bases
In most cases, these substances are used to adjust the pH of the cooling water. This allows optimization of the efficiency of other additives.
Usage overview

= median, conc = concentration in mg/L.
The use of acids and bases amounts to a total of at least 31,286 kg/year (approximately 5.8% of the total use of additives). Acids and bases are basically toxic. However, when diluted with surface water, acids and bases are neutralized and strongly diluted, which reduces toxicity. The adverse effects on the quality of the surface water are therefore small.
2.6 Other Corrosion Inhibitors
These additives are added to cooling water to slow down the corrosion rate of metal. The formed salts deposit on the surface of the metal, creating a protective layer. The most commonly used corrosion inhibitors are silicates, phosphates and phosphonates, and are poorly degradable. The average total use of other corrosion inhibitors is 269 kg/year (approximately 0.04% of the total use of additives). The substances used vary from toxic to little harmful.
Usage overview
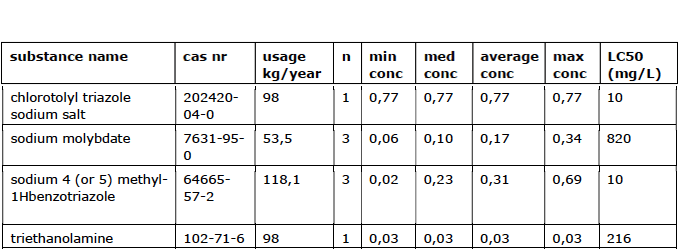
= conc. in mg/L.
2.7 Descaling and De-Chlorinating Agents
These additives are added to cooling water to remove lime particles and (excess) chlorine from the cooling water system.
Usage overview

med = median, conc = conc. in mg/L.
Maleic acid is a dicarboxylic acid used for descaling. The LC50 for crustaceans is (42.81 mg/L).
Sodium bisulfite
This substance is used to neutralize active chlorine. When active chlorine and sodium bisulfite react, sodium sulfate and sulfuric acid are formed. Sodium bisulfite is not very toxic. The EC50 of sodium bisulfite for invertebrates is 1.166 g/L.
The total use of descaling and de-chlorinating agents is 37,444 kg/year (approximately 6.6% of the total use of additives). The substances and degradation products are not very toxic.
2.8 Surfactants
Surfactants (surface-active substances) are used as a cleaning agent.
Significant amounts of these substances might be released into the environment. Degradability varies and depends largely on the solubility and adsorption of the substance. Surfactants with a C18 carbon chain or less are completely degraded relatively quickly (<20 days). The degradation rate increases with a longer carbon chain and also depends on the degree of dispersion of the substance (Vollebregt et al. 1997). Table 8 gives an overview of the types of surfactants used and the LC50.
Usage overview
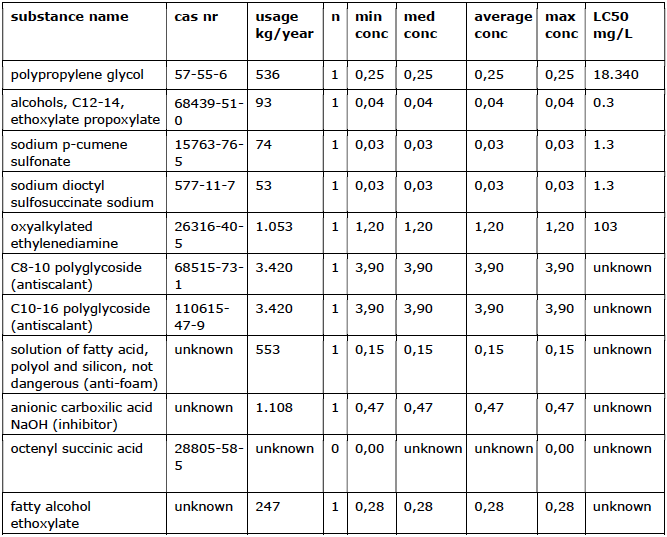
in mg/L.
Environmental properties

The total use of surfactants is at least 10,557 kg/year (approximately 2% of the total use of additives).
2.9 Anti-foaming agents
The purpose of these products is to reduce foaming in the water. There are various mixtures that have a foam-preventing or foam-breaking effect. These blends are often formulated to achieve specific results, which may vary by application. Anti-foaming agents often contain a mix of water, oil, active ingredients, and some other
auxiliary substances (for example, stabilizers).
Usage overview
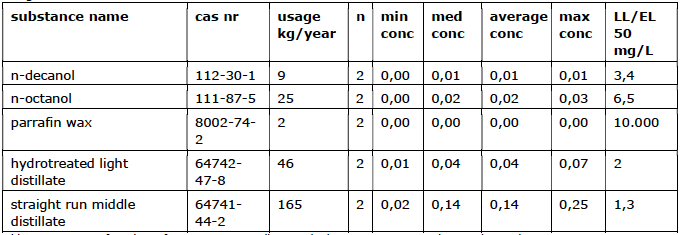
conc = conc. in mg/L
N-Decanol
This is a colorless liquid that evaporates quickly. The substance dissolves poorly in water. It is a toxic substance that breaks down quickly in water. The lowest demonstrated EC50 of this substance is 1.5 mg/L (algae and bacteria).
N-Octanol
A colorless liquid that is used for, among other things, defoaming. This material breaks down quickly in water. The lowest demonstrated EC50 concentration of this substance is 6.5 mg/L (algae and bacteria).
Parafine wax
This is a petroleum product and dissolves in both water and oils. The LL50 is 10 g/L (fish).
Hydrotreated light distillate
This is a petroleum product that dissolves poorly in water. The EL50 is 2.0 mg/L (invertebrates).
Straight run middle distilate
This is a petroleum product that is poorly soluble in water. The EL50 is 1.3 mg/L (vissen).
he use of anti-foaming agents amounts to a total of 247 kg/year (approximately 0.43% of the total use of additives by the companies). Most anti-foaming agents used are toxic. Given the low volumes of substances used and the rapid degradability of these substances, the risks for the quality of the surface water are
negligibly small.
2.10 Other Applications/Unknown Substances
The overview below (table 11) shows the substances for which the information is not complete. It concerns substances of which the substance is not indicated, nor in what quantities it is used and/or what the function of the substance is.
Usage overview
= median, conc = conc in mg/L.
The total use of other and unknown substances is at least 103,250 kg/year (at least 18% of the total use of additives by the companies). The quantities, among other things, of the surfactant modified ethoxylate anionic surfacant are unknown. This surfactant actually belongs to a different substance group. However, it was included here because nothing else is known about this substance.
3. Field Research on Biocides
In 1999, the former RWS/RIZA conducted field research into biocides (oxidative and non-oxidative agents) in recirculating cooling systems (Baltus et al., 1999). This chapter briefly summarizes the findings from this study. A comparison is also made with the industrial discharges and substances that were discussed in this study.
3.1 Oxidative Agents
Active chlorine
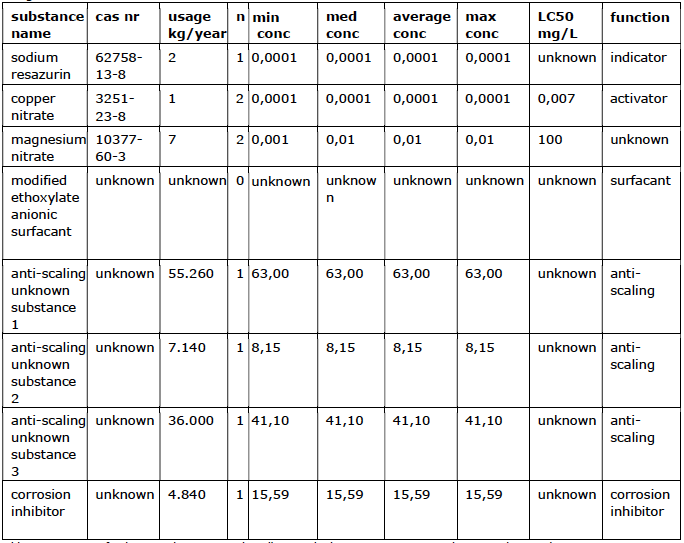
The results of the field study show that there is a clear relationship between the development of the concentration of free chlorine in the blowdown water and the percentage of light inhibition of luminescent bacteria (see figure 2). A temporarily increased concentration of free chlorine is effective to prevent bacterial growth. The study shows that sodium hypochlorite is particularly effective at temporarily high
doses. Partly because it reacts away quickly. At a concentration of 2.75 mg/L of free chlorine in the cooling water system, a reduction to 87% has been demonstrated after 30 minutes. After 30 minutes, the concentration of chlorine in the cooling water and the inhibitory effect on the bacteria drop sharply (see figure 2).
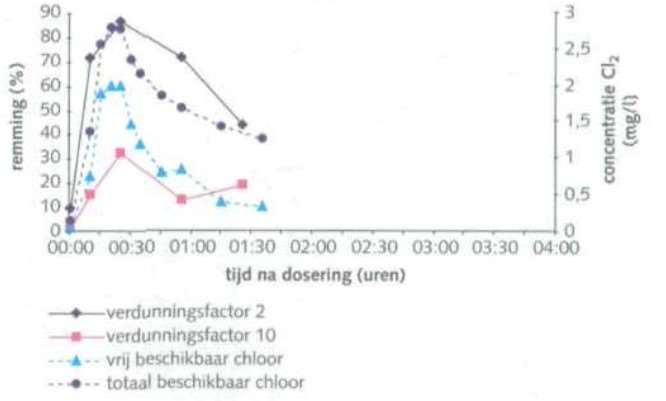
bromochloro-5,5-dimethylimidazolidine-2,4-dione (BCDMH)
In water, this substance quickly decomposes into HOBr, HOCl and dimethylhydantoin. HOBr is the primary biocide and bromamines are also produced as a by-product (Berbee, R.P.M., 1996). Figure 3 shows the biocidal effect of BCDMH in a recirculating cooling system (Baltus 1999).
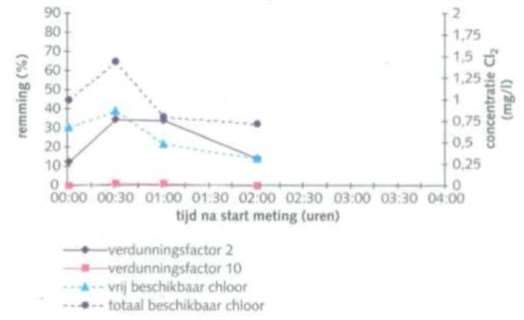
Figure 4 shows the biocidal activity of sodium hypochlorite together with sodium bromide. There is a decrease in both the concentration of active chlorine and the inhibition of luminescent bacteria over time.
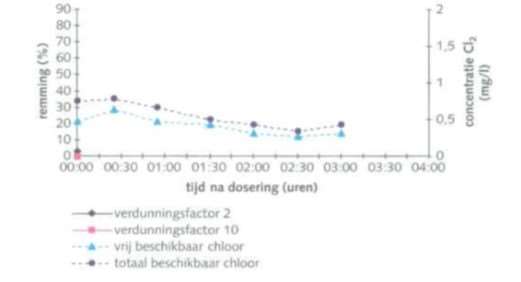
Table 12 contains an overview of the concentrations of active chlorine that were measured in the cooling systems in the RIZA study.

The different oxidative biocides described for the twelve companies correspond in terms of type of substances to those in the RIZA study. Table 12 shows average dosed concentrations. In practice, there will be peak concentrations of free chlorine. The data in the RIZA field survey gives an idea of what is actually found in practice. Shock dosage of bleach (free chlorine) has a strong effect on bacteria. It is highly toxic. Fortunately, the concentrations decrease quickly. The different figures do show that, even at low concentrations of free chlorine, inhibition of the luminescent bacteria still occurs.
3.2 Non-Oxidative Biocides
To date, little data is available regarding the use and concentration of biocides in cooling water systems. This has only been researched in the aforementioned RIZA study (Baltus, 1999). The results of this study show that different types of oxidative and non-oxidative biocides are used in the Netherlands. Table 13 provides an overview of the concentrations of non-oxidative biocides from the RIZA study (Baltus, 1999).

Figure 5 shows how the relative toxicity of the wastewater measured with luminescent bacteria decreases over time. For some products, toxic waste water (isothiazolines and glutaraldehyde) remains for some time after application. For DBNPA, the decrease in toxicity was more pronounced. In the practice of licensing,
RWS sees regular use of isothiazolines and DBNPA. DBNPA is the product used at one of the twelve companies.
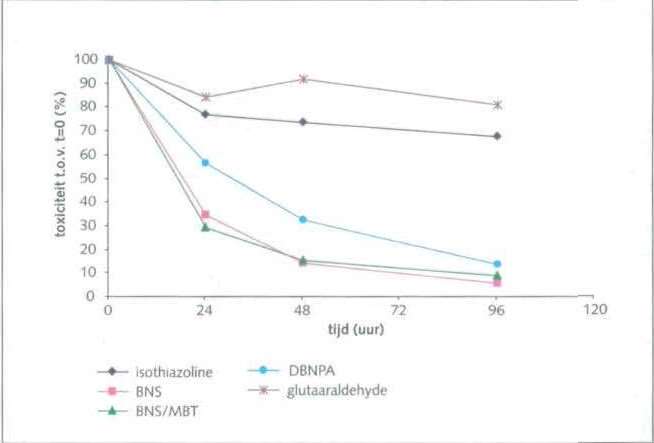
Figure 6 shows the detailed results of the readings from the RIZA field study of DBNPA. There is an increase in concentrations after dosing and a slow decrease thereafter. This substance is also used by one of the companies in this study.

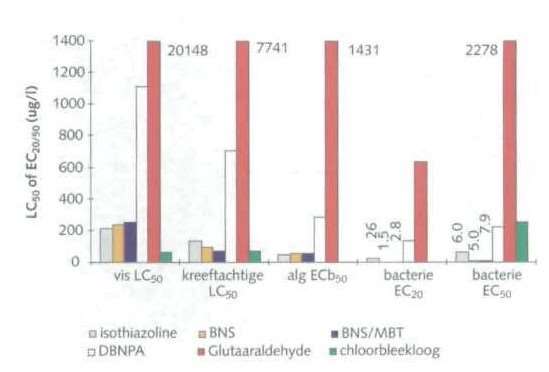
Figure 7 shows an image of the measured toxicity from the RIZA field study for the various non-oxidative biocides. It is clear that the agents used are very acutely toxic. In view of the concentrations found in the blowdown of the cooling towers (see Figures 2 to 6), it is important to limit the adverse effects of these agents on surface water by detoxification. Sodium bisulfite is often used for this.
4. Toxicity of Polymers
4.1 PPG Study of Classification of Polymers
Commissioned by the Polyelectrolyte Producers Group EEIG (PPG), studies have been carried out into the classification of polymers in cooling water additives (polycarboxylates and anionic polyacrylamide) according to the 2016 General Assessment Methodology.
The results of these studies (Verhaar H.J.M., 2019) show that these substances can be considered as little harmful to aquatic organisms. The toxicity data are described in Table 14. These substances are water-soluble anionic polymers with a low to medium molecular weight, and do not bioaccumulate. These materials include a variety of homopolymers and copolymers and are often used as detergents or to stabilize water hardness and to keep contaminants in dispersion.
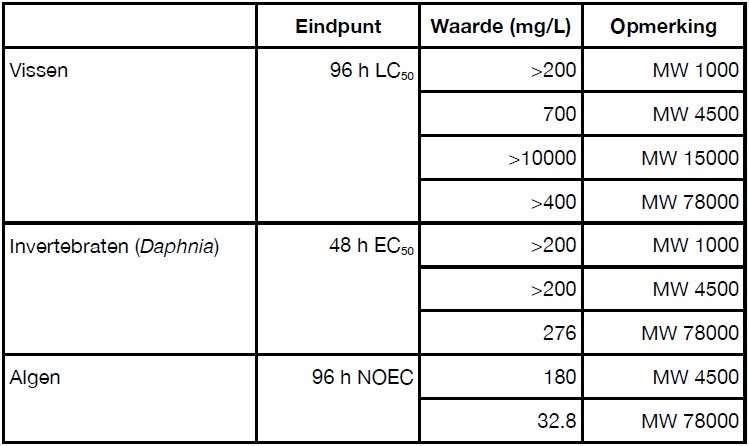
Both types of polymers are considered not readily biodegradable. PPG’s reports state that the polymers are sensitive to UV light. Once they have reached an average molecular weight of less than 10,000 g/mol by degradation, these substances are further degraded by biodegradation and mineralization.
Based on these findings, it is stated that these polymers can be classified with a water hazard class of B(4) (little harmful to aquatic organisms).
5. Evaluation
Phosphates and Phosphonates
The concentrations of phosphates and phosphonates used differ widely (0.01 mg / L 19.61 mg / L). The toxicity of the substances is not high. However, the substances are often very persistent. According to this study, the phosphonate ATMP is used by one company. This substance decomposes to AMPA. This is the same substance that occurs when the herbicide glyphosate is converted. Because AMPA can also come from cooling water systems, this can lead to a discussion with the drinking water companies whether the legal standard of 0.1 μg/L for pesticides applies.
Polymers
The average concentrations of polymers per company differ widely. The range lies between 0.22 mg/L and 197 mg/L, and the median concentration is 4.68 mg/L. The results of this study show that, by mass, about 45% of the additives are polymeric. The companies from this study collectively discharge about 261 tons of polymers into the surface water. They form a small part (rough estimate about 30%) of the total cooling water discharge in the Netherlands.
On behalf of the OSPAR (Assessment document of land-based inputs of microplastics in the marine environment), an inventory was carried out in 2017 into the origin of microplastics in surface water. The National Institute for Public Health and the Environment (RIVM) has conducted the Dutch part of this study (Verschoor, A.J., De Valk E., RIVM report 2017-0193). Microplastics are defined by ECHA as “very small (typically smaller than 5 mm) solid particles composed of mixtures of polymers (the primary components of plastics) and functional additives; they may also contain residual impurities from their manufacture”. (European Chemicals Agency (ECHA)). Cooling systems do not produce solid but dissolved/dispersed “microplastics”. Technically speaking, however, they are not microplastics. But, when a comparison is made with the quantities of microplastics (figure 8), there is a considerable amount of product that enters the surface water every year.

0193)
It is unclear what the adverse effects of large amounts of polymers in surface water are. Think of organisms that may recognize polymers as food.
The 2019 PPG study states that polymers degrade to shorter polymers under the influence of ultraviolet (UV) light. These shorter polymers are further broken down by micro-organisms. However, it is not clear whether these processes provide complete degradation.
Much of the UV light spectrum is hardly absorbed in pure water (Kirk 1994). However, it is unclear to what extent other factors influence the transmittance of UV light in surface water. The extent to which light penetrates into surface water depends strongly on the concentrations of silt, algae and humic acids in the water
(Stomp et al 2007). Dutch surface waters and coastal waters contain predominantly high concentrations of these substances and organisms. As a result, only part of the surface water is available for light degradation by UV light. It is also not known whether polymers float in surface water or tend to sink and/or form deposits with calcium and magnesium salts. When polymers sink or precipitate, the degradation by UV light is very low in practice. Polymers with a molecular weight of less than 10,000 g/mol can be degraded by mineralization, but this takes a relatively long time. The degradation rate of polycarboxylates due to mineralization is also low.
Oxidative Biocides
Average concentrations of sodium hypochlorite use vary widely. Overdosage can result in high concentrations of haloforms in the surface water. The dosage of sodium hypochlorite also strongly depends on factors such as the presence of easily oxidizable (in)organic compounds and nitrogen-containing compounds. It is very important that the surface water taken up in the cooling towers is pretreated in order to minimize the quantities of suspended matter.
Non-Oxidative Biocides
One of the companies uses DBNPA. The water hazard of DBNPA, but also of the other biocides, is high. A substance such as DBNPA, but also other non-oxidative biocides, is usually only used when active chlorine no longer works. The results from the previous RIZA study indicate that high concentrations of biocides are used in practice (mg/l level). It is important that discharges of these substances are detoxified with sodium bisulfite (Dow).
Antiscalants
It is striking that many cooling tower systems use antiscalants that are partly degradable. This leads to enrichment of the cooling water with nutrients, which in turn stimulates growth.
Other Substances
Sodium tetraborate is used in one company. This substance is considered to be a substance of very high concern (ZZS). It is recommended to check whether this product is really essential.
Other Corrosion Inhibitors
These substances are used in small quantities and are not very harmful to aquatic organisms. It is unclear whether these substances are broken down (completely) in surface water in a short time.
Unknown Substances
18% of the additives used are not known or insufficient data is available to what extent these additives pose a risk to water quality. This means that the 12 companies per year collectively discharge more than 100 tons of cooling water additive of which the water hazard cannot be sufficiently demonstrated.
6. Alternatives
This chapter describes two options for reducing the use of additives in open recirculating cooling systems.
6.1 Vortex Technology
Vortex technology is a physical method to reduce calcification of cooling water systems. When using this technique, it is no longer necessary to use cooling water additives for this purpose.
Operation
A whirlpool is created in the recirculating cooling system. This creates cavitation forces through which gases escape from the water. The lime particles that are formed during this process prefer to dissolve finely dispersed in the water. As a result, lime particles do not adhere to the inner wall of a cooling water system. This also increases the cooling efficiency of the installation.
The dispersed lime is in most cases captured. However, it is also possible to discharge it into surface water. As a result, the use of polymers and phosphonates can be dispensed.
Application Possibilities
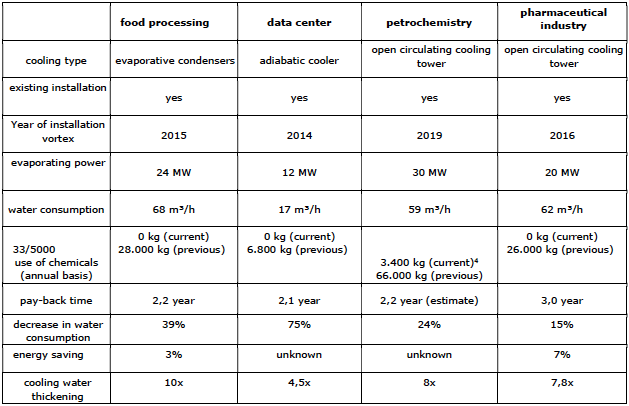
This technology has been used in the Netherlands since 2012. In 2019 there are approximately 70 circulating cooling systems that use the Vortex technology. These are both small and large cooling systems from various industrial sectors. Think of the food industry, data centers and the (petro)chemical sector. The equipment is
easy to install on both new and existing cooling systems. Most current applications have been applied to existing systems.

Case Studies
Table 15 shows four examples. In the selection of companies, the type of industry where the vortex technology is applied has been taken into account, and the size (small and large flow rates) has been considered. The data shows that the use of chemicals has decreased to almost 0.
Risks
The risks of start-up problems are low. A problem that can occur is that the system becomes clogged because the lime filtration system is not properly adjusted. No installation has been taken back until October 2019. In addition, the cooling system is checked beforehand and cleaned if necessary.
Pay-Back Time
The payback time of the installation is a maximum of three years. After this period, the financial savings compared to chemical water treatment vary between 30 and 80%.

Other Techniques
n addition to the vortex technology, UV lamps are also used to control organisms in the cooling water system. If this technique is not sufficient, electrolysis can also be used. Electrolysis causes the chlorides present in the water to convert to active chlorine. This has an inhibiting effect on organisms
6.2 Cooling Water Pre-Purification
If the cooling water is as clean as possible, in many cases fewer chemicals are needed to optimize the quality of the cooling water. An example of this is to remove ammonium from cooling water in advance. As a result, it is expected that the amount of sodium hypochlorite required can be much lower. This is, for example, applied to the cooling water units of one data center.
7. Recommendations
It is recommended to describe the ingredients of cooling water additives listed in safety sheets more clearly. A significant percentage of the safety data sheets examined did not contain enough information to properly assess the water hazard of these products. Insufficient data is available for at least 18% of the additives used to properly assess the adverse effects of the use of these substances on surface water. It is also recommended to pay attention to this when granting a water permit or drawing up a customized regulation based on the Activities Decree.
Large amounts of additives are used in cooling systems. A large part of these are anti-scaling agents. These are often poorly degradable substances, which are not very toxic. However, some of these resources are problematic in the production of drinking water from surface water.
A good alternative is the application of Vortex technology, eliminating the need for large quantities of chemicals to enter the surface water without treatment. The payback period of this technique is short (maximum three years). The competent authority is advised to demand low-chemical cooling water treatment from companies, or at least to investigate the options. It is advisable to include the Vortex technology in the BREF cooling water5 document when modifications for this document are made in the future.
When withdrawing cooling water with a lot of suspended matter or with many nitrogen compounds, pre-cleaning of the cooling water is important. As a result much less additives are needed, including biocides. Pre-purification of water only happens incidentally.
Practical research into biocides in cooling towers shows that through shock dosing, the concentrations of active chlorine decrease rapidly over time. For non-oxidative biocides, the toxicity is maintained for a longer time. It is recommended to ensure in the permit granting that the cooling system blowdown must be closed for several hours during dosing. These substances are highly toxic and it should be considered to demand standard detoxification with sodium bisulfite (DOW chemicals).
8. Bibliography
- Adriaensen R. et al. (2001). Aqua Nederland positioning paper “koel en ketelwateradditieven”;
- anonymous (1991) Betz Handbook of industrial water Conditionering. Betz laboratories Inc Trevosa PA.;
- Baltus, C.A.M.(1999). Acute toxiciteit van koelwaterlozingen uit recirculatiesystemen. Riza rapport 99.025;
- Baltus, C.A.M. Berbee, R.P.M, (1996), Het gebruik van biociden in recirculatiesystemen Riza rapport 96.036;
- Berbee R.P.M. (1997), Hoe omgaan met actief chloor in koelwater? Riza rapport 97.077;
- DOW, 2020 Chemicals Spills, Deactivation and Disposal of Glutaraldehyde: Safe Use and Handling Guide,
http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0936/0901b80380936cd5.pdf?filepath=microbial/pdfs/noreg/253-03304.doc&fromPage=GetDoc; - European Chemicals Agency (ECHA). (sd).https://echa.europa.eu/nl/hottopics/microplastics. Opgeroepen op juli 23,2019;
- Kalf D.F., Berbee R.P.M., (2002) Bronnen van AMPA op rij gezet;
- Ulrich, E., Gartiser, S. (2001). Einsatz umweltverträglicher Chemikalien in der Kühlwasserkonditionierung ;
- Verhaar A.J.M. (2019) Polycarboxylaten Klassificatie volgens de Algemene Beoordelingsmethodiek (ABM);
- Verhaar A.J.M. (2019) Anionogeen Polyacrylamide Klassificatie volgens de Algemene Beoordelingsmethodiek (ABM);
- Verschoor A.J., De Valk E. (2018) Potential measures against microplastic emissions to water RIVM report 2017-0193;
- Vollebregt, L.H.M., Westra, J. (1997) Milieu effecten van Antiscalantn.
Appendix 1
Abbreviations
- EC50: Effect Concentration 50. The concentration at which half of the test organisms show an effect after a period of time.
- HOBr Hydrogen hypobromite
- HOCl: Sodium hypochlorite
- LC50: Lethal Concentration 50. The concentration at which half of the test organisms have died after a certain period of time.
- MSDS: Material Safety Data Sheet.
- NaBr: Sodium bromide
- RIZA: National Institute for Freshwater Management and Wastewater Treatment (Rijksinstituut voor Zoetwaterbeheer en Afvalwaterbehandeling)
- RWS: Department of Water Management (Rijkswaterstaat)
- UV-light: Ultra violet light
Appendix 2
Overview of the additives per company
Company 1 (14,50 m3/hour)

Company 2 flow rate (240 m3/hour)

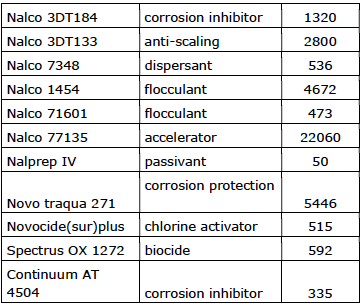
Company 3 (36,4 m3/hour)

Company 4 (15 m3/hour)

Company 5 flow rate (400 m3/hour)
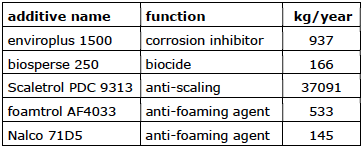
Company 6 flow rate (33 m3/hour)

Company 7 (268 m3/hour)

Company 8 flow rate (100 m3/hour)

Company 9 flow rate (14,52 m3/hour)
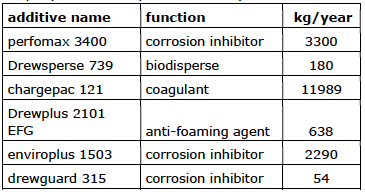
Company 10 (17,89 m3/hour)


Bedrijf 12 (1,4 m3/hour)

Appendix 3
Overview of used ingredients per company
Company 1
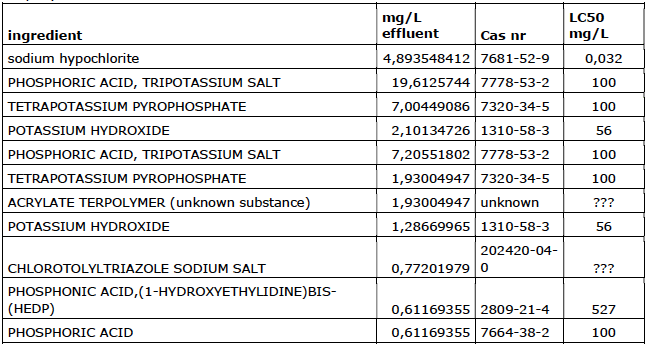
Company 2
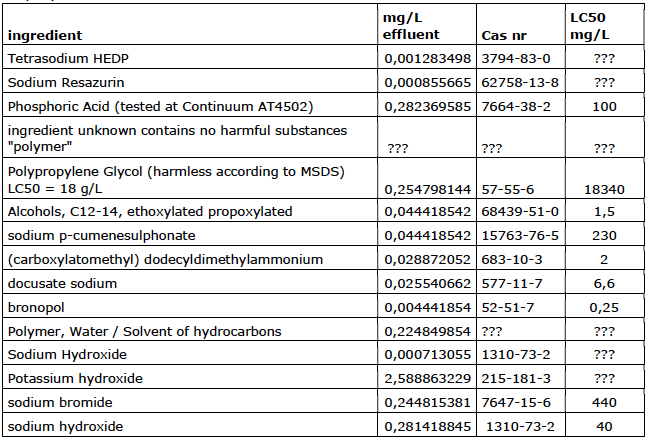

Company 3

Company 4
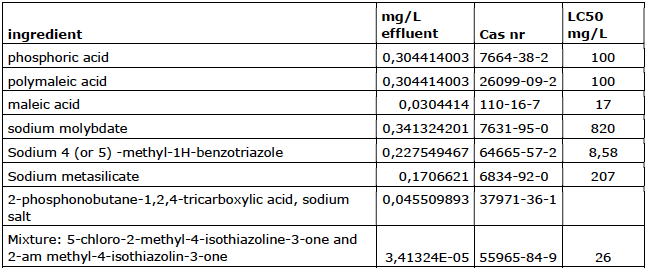
Company 5
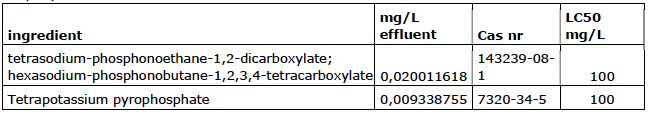
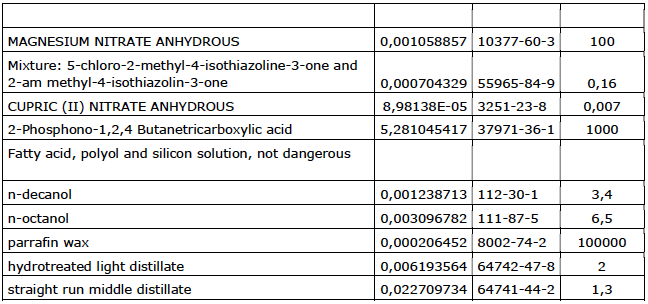
Company 6

Company 7

Company 8

Company 9

Company 10

Company 11

Company 12
Appendix 4
Brief description of non-oxidative biocides presumably used as preservatives for additives.
Bronopol
Is a non-oxidative biocide that is effective against a wide range of micro-organisms. Enzyme activity in the protein of the organism is blocked by forming a sulfide bridge. Bronopol is readily biodegradable and the hydrolysis of bronopol is low at pH 8 and 30 degrees Celsius.
Isothiazoline
Consists of a mixture of 2-methyl-4-isothiazolin-3-one and 5-chloro-2-methyl- 4isothiazolin-3-one (1: 3 ratio). It is a non-oxidative biocide with a broad spectrum effect. It’s effect is based on a rapid interaction of the isothiazolines with proteins in the cell, inhibiting ATP synthesis. Isothiazolines are effective at low concentrations (Berbee et. al).
This substance poses a risk to the quality of the surface water. Isothiazolines are inherently biodegradable and bioaccumulate. Research shows that approximately 95% of the isothiazoline used in an open recirculating cooling system is ultimately discharged into surface water (Baltus 1996). Toxicity is believed to be higher when isothiazoline is used in combination with antiscalants or dispersants.